TOXIC CHEMISTRY: Identifying the most dangerous pollutants in our ecosystem
- Toxic Chemistry: Identifying the Most Dangerous Pollutants in Our Ecosystem
Pollution: The presence of undesirable substance more than their threshold limit, causing the adverse changes change in the physical, chemical and biological nature of the environment along with exhibit very harmful effect on human life and other living organism is known as pollution. There may be foreign as well as naturally occurring pollutants that causes pollution.
Pollutant: Any substance, or form of energy which has the ability to pollute the environment, causing harmful effects and damaging nature in general. It might affect natural health of plants, the quality of air, human health, etc. Pollutants are either natural or manmade.
Source of pollution: The important source of environment pollution is
- Anthropogenic source (Agricultural activity, domestic activity, Industrialisation, Over population, Deforestation: Destruction of natural habitat etc.)
- Natural sources (Volcano, organic compounds from plants, suspended soils and dusts etc).
Types of pollutant:
- Chemical: Toxic metal (As, Pb, Hg etc), gaseous (NOx, SOx, O3) particulates (both organic and inorganic) , hydrocarbons etc.
- Physical: Heat, radiation and radioactive emission etc.
- Biologicals: Pathogenic organism.
Pollution also classified in to two categories:
- Primary pollutant: Primary pollutants are the pollutants, which are emitted directly from identifiable sources.
eg CO2, SO2, NO, NO2 Carbon monoxide, etc,
- Secondary pollutant: Secondary pollutants, are those which are produced in the atmosphere when certain chemical reactions take place among primary pollutants eg SO3, HNO3, H2SO4 peroxy acetylnitate etc.
Photochemical smog:
“Smog” is a term originally formed by the combination of of the words “smoke” and “fog. Photochemical smog is a mixture of air pollutants formed by the reaction of nitrogen oxides (NOx) and volatile organic hydrocarbons (VOCs) when they are exposed to sunlight. It is characteristic by brown hazy fumes having characteristic order of O3. It is highly oxidising due to presence of O3 and organic peroxide compound like peroxy acetyl nitrate (PAN), Peroxy benzyl nitrate etc. It leads to cracking of rubber, damage to paints, clothes and biological systems. Photochemical smog is also referred to as oxidising smog. Nitrogen oxides (the mixture of NO and NO2 together referred to as NOx) and volatile organic compounds (VOCs) are primary air pollutants, released in the atmosphere by automobiles and industrial processes. Nitrogen dioxide absorbs ultraviolet light and formation of nitric oxide and atomic oxygen takes place. Ozone is generated by the reaction of oxygen gas with this atomic oxygen. Ozone, aldehydes and peroxyacetyl nitrate so formed are thus secondary air pollutants. Photochemical smog is a mixture of primary and secondary air pollutants.
Formation of photochemical smog:
(i) Formation of ozone in lower atmosphere: At the high temperatures in a car’s combustion chamber (cylinder), nitrogen and oxygen from the air react to give nitric oxide.
IC engine
N2 +O2
2NO
2NO 2NO2
NO2 photo chemically dissociate to give atomic oxygen [O] in presence of sunlight.
h
NO2 S u n l ig h t NO + [O]
The atomic oxygen reacts with O2 to give O3 in lower atmosphere
O2 + [O] ![]() O3
O3
(ii) Generation of hydroxide radical: The atomic oxygen can react with H2O to give H2O2
h
H2O + [O] H2O2 2OH
Reaction of OH with NO2 and hydrocarbons
RCH3 +OH
RCH2 +H2O
RCH2 + O2 RCH2O 2
RCH2O 2 + NO RCH2O + NO2
RCH2O + O RCHO + HO2 HO2 + NONO2 + OH
This O H radical parcicipate in chain reaction.
RCHO + O· H
R ·C O + H2O
Acylperoxy (RCO3.) radicals has been be formed by the reaction of carboxyl radicals and oxygen atoms
Over all reaction h
Hydrocarons (RH) + NO2
Peroxyacetyl nitrate+ Peroxybenzyl nitrate
F r o m s u n l i g h t + R-CHO+ O3 + other particulates
Effect of photochemical smog:
Photochemical smog contains O3, peroxyacetyl nitatte (PAN), peroxybenzyl nitrate (PBzN), R-CHO. All these chemical cause several adverse effect on environment and quite harmful to living system.
- Effect on human health: Presence of excess ozone in lower atmosphere cause extrema fatigue, headache, chest discomfort, irritation to respiratory system and problem in central nerves system etc. PAN and PBzN are responsible for eye iiritation, formaldehyde is responsible for eye iirtation and sore throat. VOCs result in eye irritation and respiratory problems. Prolonged exposure to NO2 lowers resistance towards respiratory infections.
- Effect on plant: Ozone causes oxidative damage to plant tissue by dehydrating of plant leaf. PAN damage the leaves of plant and prevent photosynthesis. Presence of excess NO2 due to photochemical smog cause chlorosis of leaves.
- Effect on materials: Ozone of photochemical smog can damage textile, fabrics, dyes, paints polymer etc. Rubber is oxidised by ozone molecule.
Acid rain: Rainwater is naturally slightly acidic due to dissolved carbon dioxide. But in polluted environment the rain reacts with oxides of sulphur (SOx) and oxide of nitrogen (NOx) to form a mixture of H2SO4 and HNO3 and water as a result there is a decrease in pH of rain water. So the rain water having pH value lower than 5.7 is called acid rain. In acid rain H2SO4 is major contributor followed by HNO3.
2SO2 + O2 + 2H2O
2H2SO4
4NO2 + O2 + 2H2O 4HNO3
Effect of acid rain:
- The important cationic nutrientCa2+, Mg2+, etc are boundon cationic exchange soil leached out due to acid rain.

- Cause irritation on eye and skin
- Cause damage the building rocks
CaCO3 + H2SO4 ![]() CaSO4 + CO2 +H2O
CaSO4 + CO2 +H2O
- Increase the corrosion rate of metal.
Al2O3 + 6H+
2Al3+ + 3H2O ZnO + 2H+ Zn2+ + H2O
- Damage the plant leaves.
Green House effect:
CO2 gas in the atmosphere perform major role of heating up the atmosphere by trapping infrared (IR) rays from sun. The sun rays consist of UV-Visible and IR radiation. The O3 absorb most of the UV radiation and allows visible and IR radiation pass through the CO2 layer in the atmosphere. These IR causes heating effect on the earth. Consequently, if large amount of CO2 present in the atmosphere it causes greater heating up of the earth atmosphere by absorbing large amount of IR radiation. Hence the temperature of earth atmosphere rises. If concentration of CO2 is increase 0.75 ppm, then the temperature rising 0.050C. If this continue then world will go on warming up more and more which could ultimately melt glaciers, polar ice cap and flooding of many low lying areas and is known as greenhouse effect.
The other gases causing greenhouse effect are methane (CH4), nitrogen oxide (NO), chlorofluro carbon (CFCs) etc.
Cause of greenhouse effect:
Production of excessive greenhouse gases is responsible for enhanced greenhouse effect.
CO2 – Uses of petrol, diesel, CNG etc by automobile
CFCs- Use in refrigerator. CFCs absorb IR radiation.
NO- At the high temperatures in a car’s combustion chamber (cylinder), nitrogen and oxygen from the air react to give nitric oxide.
Effects:
- Sea level will rise due to rising in temperature as a result flooding will occur along worldwide and salt water will reach inland.
- The increased temperature would lead to melting of glaciers and polar ice cap (iii) Rise in surface temperature can adverse effect the food production.
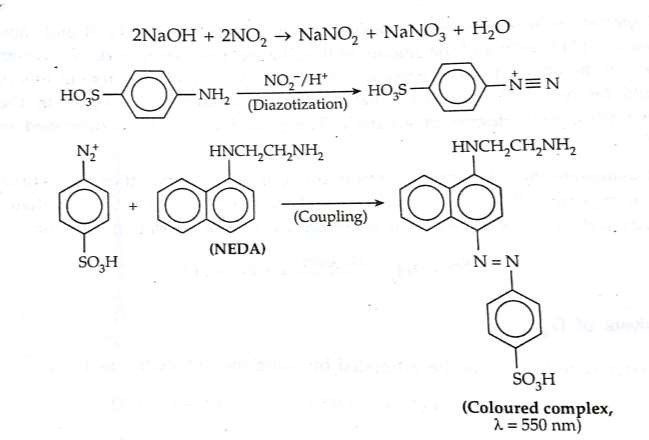
Spectrophotometric determination of NOx (Saltzmann method): This method has been used to measure the concentration of oxides of nitrogen (mainlyNO2) in a given air sample by quantitatively. In this method the NO2 is absorbed by the solution triehnaolamine in NaOH and producing nitrite ion (NO2–). The nitrite ion was convert to HNO2 in presence excess dilute acetic acid at the temperature 0 – 5 °C. The resulting HNO2 react with Sulafanilic acid to gives diazonium type of salt which further undergoes azo coupling reaction with NEDA (N-(naphthyl)ethylenediamine hydrochloride] give red colour azo dye complex. This azo dye complex has absorption maxima at 550 nm. By measuring the colour intensity of the dye the concentration of NO2 can be estimated. This method works in the range 0.01-1.5μg/mL.
In this method interference arises due to high concentration of SO2. The interference can be removed by the reaction with H2O2 to produce H2SO4 before analysis.
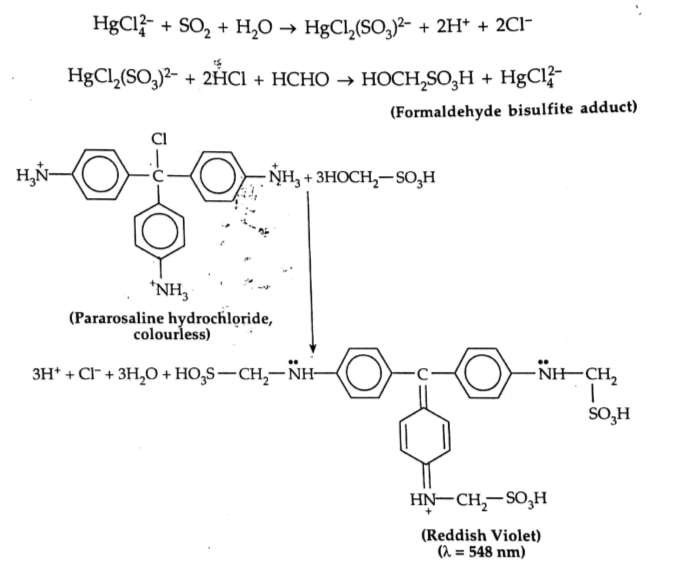
Spectrophotometric determination of SOx (West-Gaeck Method): In this method SO2 is absorb in sodium tetrachloromercurate (Na2HgCl4) solution to prevent the oxidation of SO2 to SO3 through the formation of dichlorosulfitomercurate [HgCl2(SO3)]2-. Then the solution is treated with H-CHO and with parasosaniline hydrochloride to develop a reddish violet dye having absorption maxima at 548nm. By measuring the colour intensity of the dye the concentration of SO2 can be estimated. This method works in the range 0.01-
1.5μg/mL. The reaction is carried out in dilute acidic medium (pH ~ 1.0).
In this method interference arises due NOx. The interference can be removed by adding sulfamic acid (NH2SO3H).
Spectrophotometric Determination of H2S:
| Pollution: The presence of undesirable substance more than their threshold limit, causing the adverse changes change in the physical, chemical and biological nature of the environment along with exhibit very harmful effect on human life and other living organism is known as pollution. There may be foreign as well as naturally occurring pollutants that causes pollution. Pollutant: Any substance, or form of energy which has the ability to pollute the environment, causing harmful effects and damaging nature in general. It might affect natural health of plants, the quality of air, human health, etc. Pollutants are either natural or manmade. Source of pollution: The important source of environment pollution is Anthropogenic source (Agricultural activity, domestic activity, Industrialisation, Over population, Deforestation: Destruction of natural habitat etc.) Natural sources (Volcano, organic compounds from plants, suspended soils and dusts etc). Types of pollutant: Chemical: Toxic metal (As, Pb, Hg etc), gaseous (NOx, SOx, O3) particulates (both organic and inorganic) , hydrocarbons etc. Physical: Heat, radiation and radioactive emission etc. Biologicals: Pathogenic organism. Pollution also classified in to two categories: Primary pollutant: Primary pollutants are the pollutants, which are emitted directly from identifiable sources. eg CO2, SO2, NO, NO2 Carbon monoxide, etc, Secondary pollutant: Secondary pollutants, are those which are produced in the atmosphere when certain chemical reactions take place among primary pollutants eg SO3, HNO3, H2SO4 peroxy acetylnitate etc. Photochemical smog: “Smog” is a term originally formed by the combination of of the words “smoke” and “fog. Photochemical smog is a mixture of air pollutants formed by the reaction of nitrogen oxides (NOx) and volatile organic hydrocarbons (VOCs) when they are exposed to sunlight. It is characteristic by brown hazy fumes having characteristic order of O3. It is highly oxidising due to presence of O3 and organic peroxide compound like peroxy acetyl nitrate (PAN), Peroxy benzyl nitrate etc. It leads to cracking of rubber, damage to paints, clothes and biological systems. Photochemical smog is also referred to as oxidising smog. Nitrogen oxides (the mixture of NO and NO2 together referred to as NOx) and volatile organic compounds (VOCs) are primary air pollutants, released in the atmosphere by automobiles and industrial processes. Nitrogen dioxide absorbs ultraviolet light and formation of nitric oxide and atomic oxygen takes place. Ozone is generated by the reaction of oxygen gas with this atomic oxygen. Ozone, aldehydes and peroxyacetyl nitrate so formed are thus secondary air pollutants. Photochemical smog is a mixture of primary and secondary air pollutants. Formation of photochemical smog: (i) Formation of ozone in lower atmosphere: At the high temperatures in a car’s combustion chamber (cylinder), nitrogen and oxygen from the air react to give nitric oxide. IC engine 2NO NO2 photo chemically dissociate to give atomic oxygen [O] in presence of sunlight. h NO2 The atomic oxygen reacts with O2 to give O3 in lower atmosphere O2 + [O] (ii) Generation of hydroxide radical: The atomic oxygen can react with H2O to give H2O2 h H2O + [O] Reaction of OH with NO2 and hydrocarbons  RCH3 +OH RCH3 +OH RCH2 + O2 RCH2O 2 RCH2O 2 + NO RCH2O + NO2 RCH2O + O RCHO + HO2 HO2 + NONO2 + OH This O H radical parcicipate in chain reaction. Acylperoxy (RCO3.) radicals has been be formed by the reaction of carboxyl radicals and oxygen atoms  Over all reaction h F r o m s u n l i g h t + R-CHO+ O3 + other particulates Effect of photochemical smog: Photochemical smog contains O3, peroxyacetyl nitatte (PAN), peroxybenzyl nitrate (PBzN), R-CHO. All these chemical cause several adverse effect on environment and quite harmful to living system. Effect on human health: Presence of excess ozone in lower atmosphere cause extrema fatigue, headache, chest discomfort, irritation to respiratory system and problem in central nerves system etc. PAN and PBzN are responsible for eye iiritation, formaldehyde is responsible for eye iirtation and sore throat. VOCs result in eye irritation and respiratory problems. Prolonged exposure to NO2 lowers resistance towards respiratory infections. Effect on plant: Ozone causes oxidative damage to plant tissue by dehydrating of plant leaf. PAN damage the leaves of plant and prevent photosynthesis. Presence of excess NO2 due to photochemical smog cause chlorosis of leaves. Effect on materials: Ozone of photochemical smog can damage textile, fabrics, dyes, paints polymer etc. Rubber is oxidised by ozone molecule.   Acid rain: Rainwater is naturally slightly acidic due to dissolved carbon dioxide. But in polluted environment the rain reacts with oxides of sulphur (SOx) and oxide of nitrogen (NOx) to form a mixture of H2SO4 and HNO3 and water as a result there is a decrease in pH of rain water. So the rain water having pH value lower than 5.7 is called acid rain. In acid rain H2SO4 is major contributor followed by HNO3. 4NO2 + O2 + 2H2O Effect of acid rain: The important cationic nutrientCa2+, Mg2+, etc are boundon cationic exchange soil leached out due to acid rain. Cause irritation on eye and skin Cause damage the building rocks CaCO3 + H2SO4 Increase the corrosion rate of metal. Damage the plant leaves. Green House effect: CO2 gas in the atmosphere perform major role of heating up the atmosphere by trapping infrared (IR) rays from sun. The sun rays consist of UV-Visible and IR radiation. The O3 absorb most of the UV radiation and allows visible and IR radiation pass through the CO2 layer in the atmosphere. These IR causes heating effect on the earth. Consequently, if large amount of CO2 present in the atmosphere it causes greater heating up of the earth atmosphere by absorbing large amount of IR radiation. Hence the temperature of earth atmosphere rises. If concentration of CO2 is increase 0.75 ppm, then the temperature rising 0.050C. If this continue then world will go on warming up more and more which could ultimately melt glaciers, polar ice cap and flooding of many low lying areas and is known as greenhouse effect. The other gases causing greenhouse effect are methane (CH4), nitrogen oxide (NO), chlorofluro carbon (CFCs) etc. Cause of greenhouse effect: Production of excessive greenhouse gases is responsible for enhanced greenhouse effect. CO2 – Uses of petrol, diesel, CNG etc by automobile CFCs- Use in refrigerator. CFCs absorb IR radiation. NO- At the high temperatures in a car’s combustion chamber (cylinder), nitrogen and oxygen from the air react to give nitric oxide. Effects: Sea level will rise due to rising in temperature as a result flooding will occur along worldwide and salt water will reach inland. The increased temperature would lead to melting of glaciers and polar ice cap (iii) Rise in surface temperature can adverse effect the food production. Spectrophotometric determination of NOx (Saltzmann method): This method has been used to measure the concentration of oxides of nitrogen (mainlyNO2) in a given air sample by quantitatively. In this method the NO2 is absorbed by the solution triehnaolamine in NaOH and producing nitrite ion (NO2–). The nitrite ion was convert to HNO2 in presence excess dilute acetic acid at the temperature 0 – 5 °C. The resulting HNO2 react with Sulafanilic acid to gives diazonium type of salt which further undergoes azo coupling reaction with NEDA (N-(naphthyl)ethylenediamine hydrochloride] give red colour azo dye complex. This azo dye complex has absorption maxima at 550 nm. By measuring the colour intensity of the dye the concentration of NO2 can be estimated. This method works in the range 0.01-1.5μg/mL. In this method interference arises due to high concentration of SO2. The interference can be removed by the reaction with H2O2 to produce H2SO4 before analysis. Spectrophotometric determination of SOx (West-Gaeck Method): In this method SO2 is absorb in sodium tetrachloromercurate (Na2HgCl4) solution to prevent the oxidation of SO2 to SO3 through the formation of dichlorosulfitomercurate [HgCl2(SO3)]2-. Then the solution is treated with H-CHO and with parasosaniline hydrochloride to develop a reddish violet dye having absorption maxima at 548nm. By measuring the colour intensity of the dye the concentration of SO2 can be estimated. This method works in the range 0.01- 1.5μg/mL. The reaction is carried out in dilute acidic medium (pH ~ 1.0). In this method interference arises due NOx. The interference can be removed by adding sulfamic acid (NH2SO3H). Spectrophotometric Determination of H2S:                |